1. Liquid metal reaction environment for synthesizing atomic-thickness metal oxides at room temperature
(A liquid metal reaction environment for the room-temperature synthesis of atomically thin metal oxides)
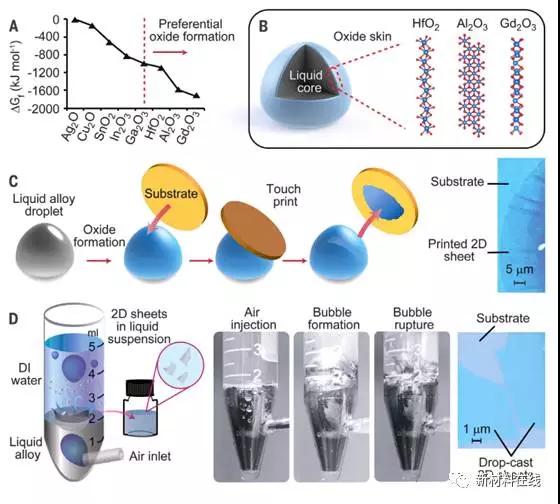
Two-dimensional (2D) oxides have a wide range of applications in electronics and other technologies. However, many oxides are not easily synthesized into 2D materials using conventional methods. Zavabeti et al. used a non-toxic gallium-based eutectic alloy as a reaction solvent and added the metal required for alloying to the melt. On the basis of considering thermodynamics, the composition of self-limiting interface oxides was predicted. Zavabeti et al. separated the surface oxide as a 2D layer on the substrate and in the suspension. This enables the production of very thin submicron grades of HfO2, Al2O3 and Gd2O3. Liquid metal based reaction methods can be used to produce 2D materials that could not be synthesized by previously existing methods. (Science DOI: 10.1126/science.aao4249)
2. Using an electric field to defibrillate a soft porous metal organic framework
(Defibrillation of soft porous metal-organic frameworks with electric fields)
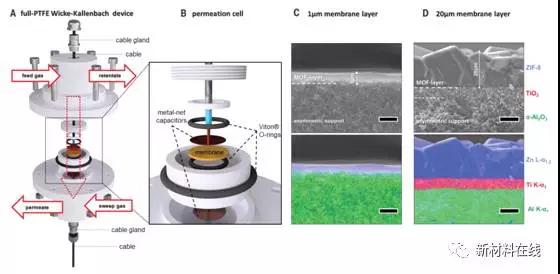
By applying an external electric field (E-field), gas is transported through the metal-organic framework film (MOF) where in-situ conversion occurs. Gas permeation conversion by electric field polarization can be explained by the conversion of the zeolitic imidazolate framework material ZIF-8 structure to a polymorph having a more rigid lattice. Permeation measurements at a DC electric field polarization of 500 volts per millimeter showed that ZIF-8 was controlled to reversibly convert to a polar polymorph, as evidenced by X-ray diffraction and ab initio calculations. The hardening of the crystal lattice results in a reduction in gas transport through the membrane and an increase in the performance of the molecular sieve. Dielectric spectroscopy, polarization and æ°˜ NMR studies reveal the low frequency resonance of ZIF-8, which Knebel et al. attributed to lattice flexibility and joint motion. At the time of electric field polarization, defibrillation of different lattice motions was observed. (Science DOI: 10.1126/science.aal2456)
3. Rotating and linear molecular motors driven by chemical fuel pulses
(Rotary and linear molecular motors driven by pulses of a chemical fuel)
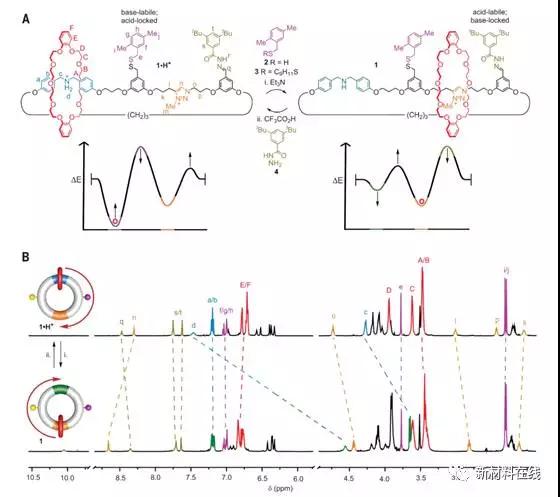
Many biomolecule motors catalyze the hydrolysis of chemical fuels (such as adenosine triphosphate) and use the released energy to guide motion through an information ratcheting mechanism. Erbas-Cakmak et al. describe chemically driven man-made rotary and linear molecular motors that operate through fundamentally different types of mechanisms. The directional rotation of the [2]- and [3] soda gas rotary molecular motors, and the linear molecular pump transfer matrix out of equilibrium, are caused by acid-base oscillations. These changes simultaneously alter the stability of the binding site affinity and obstacles in the orbit, forming an energy ratchet. Linear and rotary molecular motors are driven by an aliquot of the chemical fuel trichloroacetic acid. A [2] soda hydrocarbon rotary motor, a single fuel pulse can produce 360° unidirectional rotation of up to 87% of the crown ether. (Science DOI: 10.1126/science.aao1377)
4. MOF-derived Co nanoparticles catalyze amine synthesis
(MOF-derived cobalt nanoparticles catalyze a general synthesis of amines)
The development of alkali metal catalysts remains an important chemical research goal for the synthesis of pharmaceutical related compounds. Jagadeesh et al. reported that graphite shell-coated Co nanoparticles are very effective for reductive amination catalysts. Their convenient and practical preparation requires template assembly of a cobalt amine dicarboxylic acid metal organic framework (MOF) on carbon and subsequent pyrolysis in an inert atmosphere. The resulting stable and reusable catalysts are effective for the synthesis of primary, secondary, tertiary and N-methylamines (more than 140 samples). The reaction readily couples the available carbonyl compound to ammonia, amine or nitro compounds and molecular hydrogen under industrial conditions. This provides a cost-effective method for the synthesis of many amines, amino acid derivatives and more complex drugs. (Science DOI: 10.1126/science.aan6245)

5. A flexible metal-organic framework with high density sulfonic acid sites for proton conduction
(A flexible metal–organic framework with a high density of sulfonic acid sites for proton conduction)
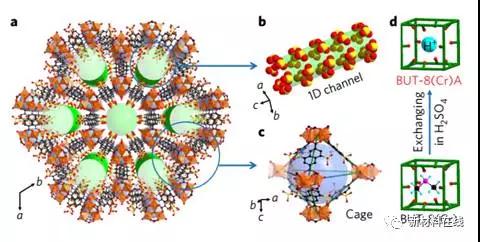
Designing stable electrolyte materials with high proton conductivity, making them useful in proton exchange membrane fuel cells, remains a significant challenge to this day. Most materials have good electrical conductivity at high relative humidity (RH), but the conductivity is significantly reduced at low relative humidity. Yang et al. reported that the chemically stable, structurally flexible metal-organic framework (MOF) BUT-8(Cr)A is a three-dimensional framework with one-dimensional channels, and that high-density sulfonic acid (-SO3H) sites are arranged in The channel surface is used for proton conduction. They propose that combining their flexible nature with their -SO3H sites allows BUT-8(Cr)A to adapt its framework in different humid environments, thus ensuring a water molecule-mediated pathway for smooth proton conduction. Compared to other MOFs, BUT-8(Cr)A has a high proton conductivity of 1.27×10-1S•cm-1 at 100% RH and 80°C, and it can be maintained over a wide range of RH and temperature. Certain high proton conductivity. (Nature Energy DOI: https://doi.org/10.1038/s41560-017-0018-7)
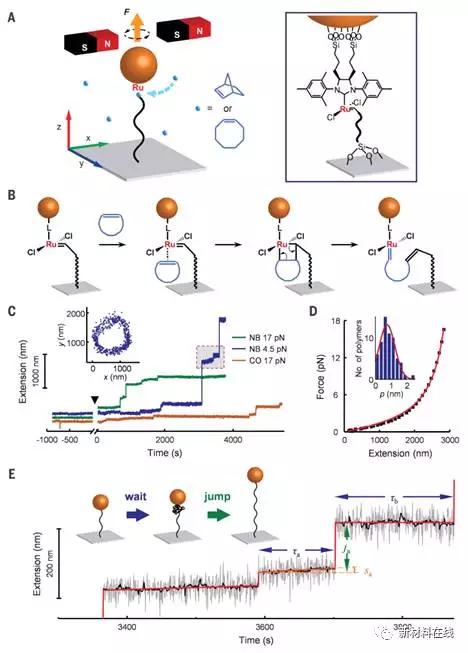
6. Single polymer growth kinetics
(Single polymer growth dynamics)
In chain-growth polymerization, chain lengths continue to grow, reaching thousands of subunits. However, the real-time dynamics of chain growth are still unknown. Using magnetic tweezers, we can visualize real-time polymer growth at the single polymer level. For open-loop metathesis polymerization, Liu et al. found that the elongation of the polymer grown under tension does not continue to increase, but rather shows the pace of waiting and jumping. These steps can be attributed to the formation and unwinding of conformational entanglements from newly introduced monomers, the main features of which can be summarized by molecular dynamics simulations. These entangled structures appear to play a key role in determining the rate of polymerization and the dispersibility between the various polymers. (Science DOI: 10.1126/science.aan6837)
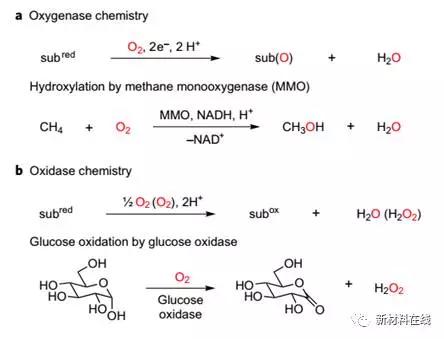
7. Oxidase catalyzes the production of high-valent iodine intermediates by oxygen consumption
(Oxidase catalysis via aerobically generated hypervalent iodine intermediates )
The development of sustainable oxidation chemistry requires a method that can use O2 as a terminal oxidant. Oxidase catalysis, where O2 is used as a chemical oxidant, does not require the introduction of oxygen into the reaction product, which will allow for the binding of different substrate functionalization chemistries to O2 reduction. The direct use of O2 is influenced by inherent challenges, including the triplet ground state of O2 and the different electronic inventory of four-electron O2 reduction and two-electron substrate oxidation. Maity et al. made high-valent iodine reagents (a widely used class of selective two-electron oxidants) from O2. This process aerobicly produces a multivalent iodine reagent for a wide range of substrate oxidation reactions by preventing the reaction intermediate of the aldehyde self-oxidation. The use of an aryl iodide as an aerobic oxidizing medium allows the construction of an oxidase catalytic platform to directly couple the substrate oxide to oxygen reduction. Maity et al. anticipate that aerobic high-valent iodine reagents will expand the range of aerobic oxidation chemistry in chemical synthesis. (Nature Chemistry DOI: 10.1038/NCHEM.2873)
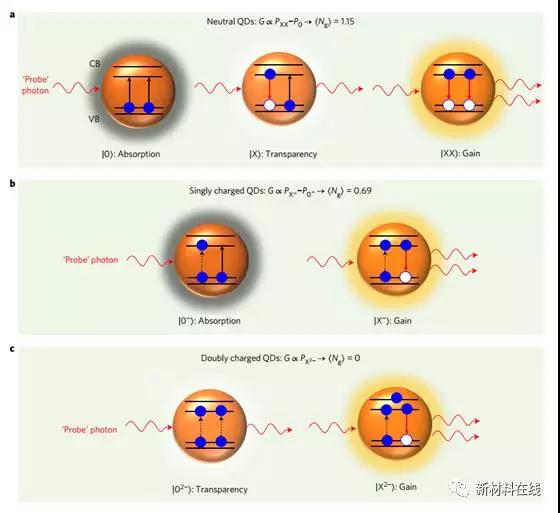
8. Using zero-threshold optical gain with charged semiconductor quantum dots
(Towards zero-threshold optical gain using charged semiconductor quantum dots)
Colloidal semiconductor quantum dots are very attractive materials for implementing solution processing lasers. However, applications as optical gain media have become complicated due to non-uniform degeneracy with edge states. Because more excitons are needed to achieve the laser emission state. This increases the laser threshold and results in a very short optical gain lifetime limited by non-radiative Auger recombination. Wu et al. have shown that these problems can be solved, at least in part, by using non-neutral but negatively charged quantum dots. By applying light doping to quantum dots specially designed for the retarded Auger effect, Wu et al. demonstrated that the optical gain threshold is significantly reduced due to pre-existing carrier suppression of ground state absorption. In addition, by injecting approximately one electron per quantum dot, the threshold for amplifying spontaneous emission is reduced by more than two times to the level of sub-single exciton. These measurements show the feasibility of achieving a "zero threshold" gain by completely blocking the edge state of the band with two electrons. (Nature Nanotechnology DOI: 10.1038/NNANO.2017.1
The Thermometer that Sanan Technology mainly runs is the Infared Thermometer for Humans.
The classifications of the Infared Thermometer
The Infrared Thermometer are mainly divided into the Contact Infrared Thermometer and the Non-contact Infrared Thermometer(Thermometer Non Contact).
As for the Contact Infrared Thermometer, the Ear Thermometer is commonly used.
The using way of the Ear Thermometer:
1. Gently straighten the ear canal;
2. Insert the temperature measuring head into the ear canal;
3. Press the upper end of the temperature measurement for one second;
4. The Accurate body temperature can be read from the LCD screen;
Safe and secure. And it is safe even if the whole family shares if only we replace the protective rubber cover when using it(to avoid bacterial infection).
While, as for the Non-contact Infrared Thermometer(Thermometer for Fever), the most common type is the Forehead Thermometer.
The using way of the Forehead Thermometer:
1. Point the probe at the forehead;
2. Press the measuring button
3. The measurement data can be obtained in just a few seconds
Very suitable for the patients with acute and serious illness, the elderly, infants and children. However, in the early stage of use, users may get several different measurement data because they are not familiar with this operation method. Generally speaking, the measured maximum value is the desired data. And the users will be more satisfied with this kind of thermometer as they get familiar with it.
All in all, the advantages of infrared thermometers(Thermometer Body Temperature) are convenient, simple, fast, and quite accurate to collect body temperature, and its disadvantage is that it can be easily affected by the the ambient temperature, and in this case the error will be large. Here are some tips about the thermometers as follows, hope it helps when you choose and use a thermometer.
Tips:
1. People with ear diseases such as the external otitis and otitis media should not use the ear thermometer.
2. Do not use the ear thermometer when the ear holes are wet after swimming or bathing.
3. When the person under test comes from a place where the temperature is very different from the temperature of the environment, he/she should stay in the measurement environment for at least 5 minutes to make the 2 temperature data becomes similar and then measure the body temperature.
5. Using the cold compresses and other cooling measures on a fever patient will make the measurement result lower. Please avoid the temperature measurement in this case.
6. Please don't measure the body temperature in the places with large air currents such as the fans and air conditioners.
7. Please don't measure the body temperature in direct sunlight.
8. We advice to measure about 3 times during the measurement with the interval between each measurement 3-5 seconds, and select the most displayed set of data.

Thermometer
Thermometer, Thermometer Non Contact, Infrared Thermometer, Thermometer Forehead, Thermometer for Fever, Thermometer Body Temperature
SHENZHEN SANAN TECHNOLOGY CO.,LTD , https://www.sanan-cctv.com